Would you like to know a bit more about the smart materials we experimented with during the workshop? There's some more information below about the experiments we did and how these materials are used. Perhaps you could come up with an invention or a clever way of using one of these materials! We'll start of with the Polymorph as this is the one in your take-home packet ...

To form this plastic into a mouldable state, hot water is needed, ideally at close to boiling point. Be careful with boiling water, particularly if children are involved in experiments,
Pour some boiling water into a coffee cup (about half a cup is plenty).
Pour the sachet of pellets into the water and wait for them to turn transparent.
Pull out the clump of pellets with a spoon and form them into whatever shape you want with your hands. After a few minutes the moulded plastic will turn white again and will have solidified.
This plastic is a biodegradable polyester (plastic) with a low melting point of around 60 degrees C. It can be heated, reformed and cooled many times.
Other than its ability to be used as a prototyping tool (to make whatever creative idea you come up with!), its biodegradability opens up many other applications. It's used as sutures to stitch up wounds and as a way to deliver medication (doctors can insert a bit into the body that has been mixed up with medicines and the body will slowly absorb the medicines) - in both applications it can degrade and be absorbed by the body after it has been used.
You won't have these materials at home, but for those who are really interested in some of the geeky science behind the other amazing materials you used in the workshop, you might like to read a bit more about the materials, their uses and the experiments we did with them below ...

If heating the wire in hot water take care as well, particularly if doing this experiment with children.
Bend the spring into whatever shape you want. Then heat it up by placing it in a mug of hot water or by using a hair dryer. It should return immediately to its previously memorised state (which in this case is a coil).
This sample is one of several known alloys (mixtures of metals) which show this remarkable property. This particular one is sold under the brand name Nitinol. The 'Ni' and 'Ti' in its name gives a clue to its composition - it's made of a mixture of Nickel and Titanium. It was an accidental invention which was discovered by scientists working at the now disbanded Naval Ordnance Laboratory in the US. (NOL - the last three letters of its brand name).
When heated above a certain temperature it immediately returns to its previously memorised shape. This shape can be fixed by heating it to a carefully controlled temperature (around 400-500 degrees) for a long time.
This alloy can be woven into a cylindrical mesh to make a 'stent' which is a device that can hold open large blood vessels in the human body from the inside. In this case the transition temperature is made close to body temperature (37 C) so that when they are inserted, they try to remember their memorised state which pushes out on the inside of the vessels.
The heating can also be created by passing an electric current through the alloy itself to make an actuator - a device which converts an electric current into movement. Many cars now have valves inside them that are moved by shape memory alloys - they're typically quieter, more energy efficient and smaller than the older coil-based devices that they replace.
Shape memory alloys can also be created with a tailored "transition temperature". If this is set below the typical ambient temperature then the alloy is permanently in a 'returning to remembered shape' mode, which makes it super springy. Many eyeglass frames are created with shape memory alloys which allow them to immediately spring back into shape, even when bent far beyond the point that a normal metal could cope with.
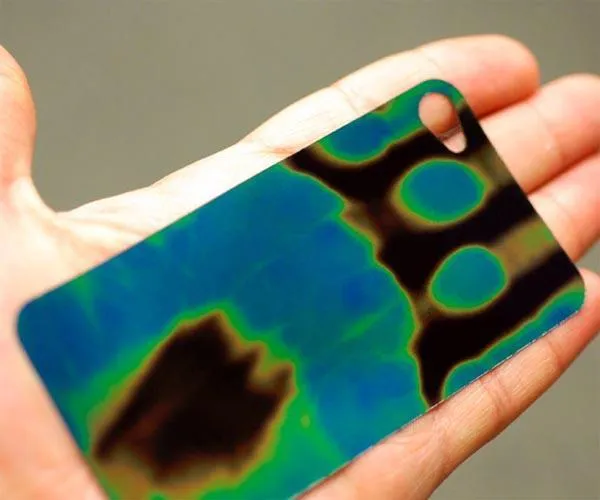
Place your fingers onto the dark side of the thermochromic panel and leave them there for around 10 seconds. When you take them away you should see that the colour of the panel has changed. Try placing other warm objects on the panel or touching the panel against them.
Many materials are naturally thermochromic - this word means it changes colour (chromic) with heat (thermo). Some materials though are particularly good at this trick. There are substances called liquid crystals that are used in this panel that can be made to change colour at many different temperatures. This product has been designed to change colour at just under the typical human body temperature (around 37 degrees C).
When formula milk is given to babies it's important that it isn't too hot. Some bottles have built in thermochromic panels on that warn you if the milk is dangerously hot. Some batteries are also made with one of these panels on the side. When you press a certain place, an electrical circuit is created which heats up the panel, showing how much charge is left in the battery.

Cut your piece of polymer in two with a pair of scissors. You'll notice that it has a protective plastic covering over one side. Place the two cut ends together, but overlapped by 5mm or so. Make sure you place the two unprotected sides together. Now press firmly with your finger to join the polymer back together. You'll need to press along the seam for perhaps 10 seconds or so. The self-healing property is stronger the harder (and longer) you press. After doing this, try to pull at the two ends of the polymer - you may be surprised how strong the join is!
There are quite a few types of self-healing materials. This one is a form of rubbery plastic called polyurethane which likes to stick to itself but not other things. There are other forms of self-healing materials that have thousands of 'microcapsules' in them - if a crack forms in the material and starts getting bigger, the microcapsules burst releasing a liquid that fills in and seals the gap.
This particular material can be used if you want to join two halves of an item together but don't want them sticking to anything else that they might touch. A similar substance is now used on some mobile phone covers (and screen protectors) - when scratches are made in the material they self-heal themselves. Some self-healing paints have also been created for use on steel structures which are based on the microcapsule approach - when they are scratched they release more liquid to cover the scratch, protecting the metal underneath from rusting.

Pour the packet of Instant Snow into a large bowl. Fill up a jug with water and pour some into bowl as well. Mix the water and Instant Snow with your hand. Now add some more water and see how much it absorbs before it gets mushy. You may be surprised! When you're done if you want to dispose of it, sprinkle some salt on top and mix it around - it will quickly turn back to a liquid and you can then pour it down the sink.
This is an amazing substance which is chemically called Sodium Polyacrylate. It's another polymer that can absorb an amazing amount of water - in this case several hundred times its own weight.
There are various forms of this polymer that can be used for all sorts of purposes. This one is sometimes used as artificial snow for indoor ski slopes (surprise surprise!) It's also used in situations where you want to absorb a large amount of a fluid (nappies!)